Recently, four members of Tianlong family, including the DNA testing kits for E. coli, Salmonella, Staphylococcus aureus, and Listeria monocytogenes have gained CE certification to be marketed in the European Union. This is another series of international approvals and certifications obtained by Tianlong after the CE certification of Tianlong’s monkeypox virus nucleic acid detection kit.

So far, Tianlong products have obtained more than 120 approvals or certifications, including EU CE, US FDA, UK, Germany, Netherlands, Korea, Japan, Ukraine, Kazakhstan, Indonesia, etc. Tianlong products have been marketed and applied in more than 80 countries and regions worldwide, which is a recognition of the quality of Tianlong products.
CE-Certified Tianlong Testing Kits
Life Sciences
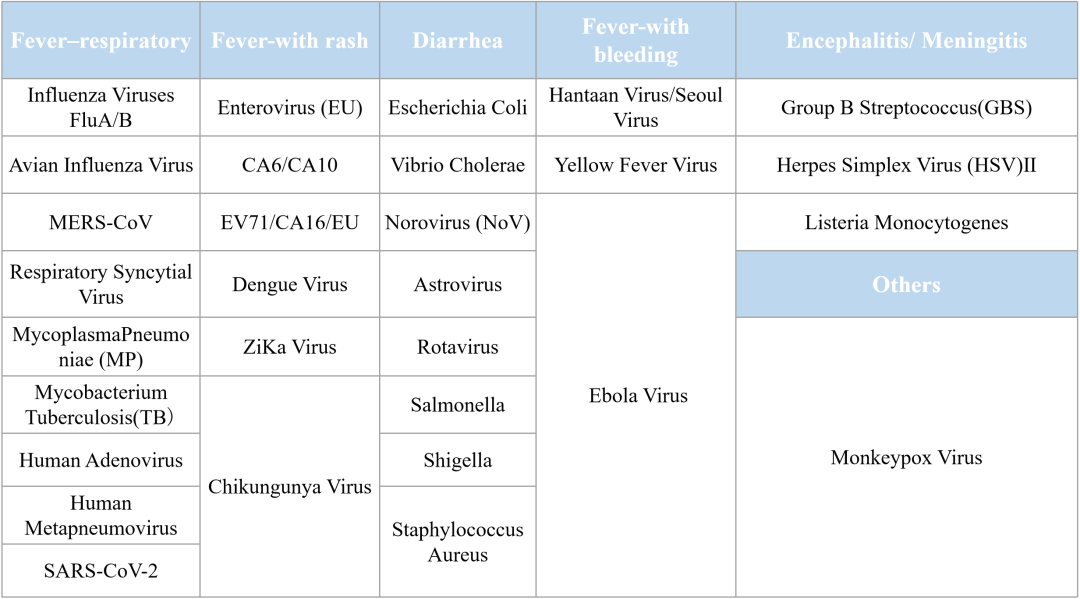
Clinical/Medical Applications
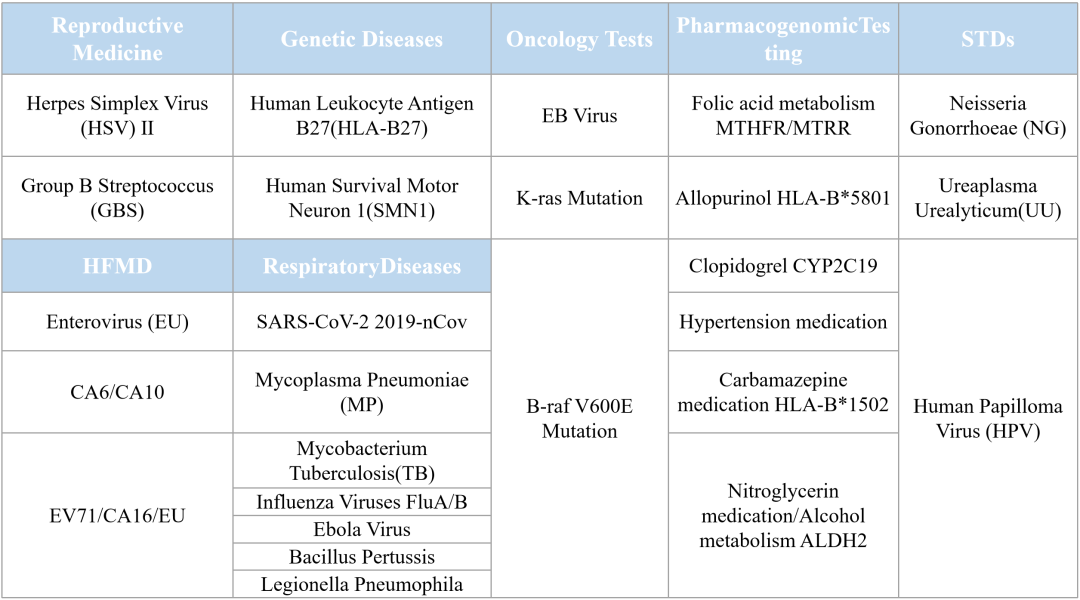
Tianlong Intelligent Manufacturing Family in Various Applications:
We Are Efficient, Precise and Comprehensive
Tianlong testing kits are high-performing, high-accuracy, and compatible with automated nucleic acid extraction, and testing instruments to comprehensively realize the automated detection of the targeted nucleic acids of the pathogens. Tianlong products have been recognized by our clients and end users in various fields, including global epidemic prevention and control, detection of infectious diseases, clinical diagnostics and precision medicine for genetic diseases and cancer, food safety, animal disease control and quarantine, etc.







In the future, Tianlong will continue to focus on global public health situations and provide first-class molecular diagnostic products and total solutions for the well-being of all.

