Suzhou TianLong Biotechnology Co., Ltd., a reagent-focused subsidiary of Xi’an TianLong Science and Technology Co., Ltd., has recently made a major breakthrough in monkeypox virus (MPXV) rapid detection technology, in collaboration with the Precision Medical Center of the Second Hospital of Nanjing and Nanjing University of Chinese Medicine. The jointly developed MIRA-CRISPR-Cas13a-based monkeypox detection system offers high sensitivity, speed, and specificity, enabling rapid viral clade identification and greatly enhancing the efficiency of outbreak monitoring and response. This achievement has been published in the internationally renowned journal Infectious Diseases of Poverty, highlighting TianLong’s strength in molecular diagnostics and translational research.
Currently, MPXV diagnosis relies heavily on epidemiological and clinical features, with confirmation via real-time PCR. However, in under-resourced regions, such as parts of Africa, limited laboratory capacity leads to many cases remaining undiagnosed. Although serological tests provide speed and convenience, they often yield false positives and fail to distinguish viral clades, hindering effective epidemic control and timely treatment.
To address these challenges, the research team developed a field-deployable MPXV detection system combining Multienzyme Isothermal Rapid Amplification (MIRA) with the CRISPR-Cas13a system. This method is easy to operate, requires no costly instruments or complex procedures, and offers high sensitivity and strong specificity. The detection limit reaches as low as 14.4 copies/mL, outperforming mainstream qPCR methods with limits around 200 copies/mL, enabling early detection of low viral load infections.
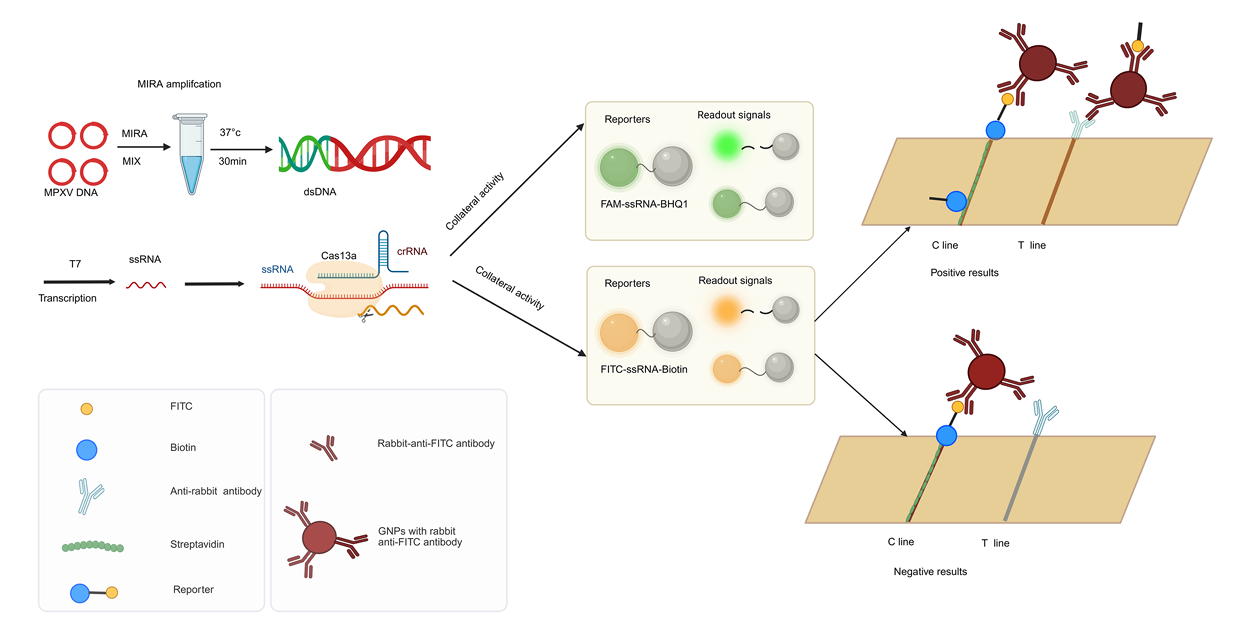
Figure 1 shows the principle of the MIRA-CRISPR-Cas13a detection system.
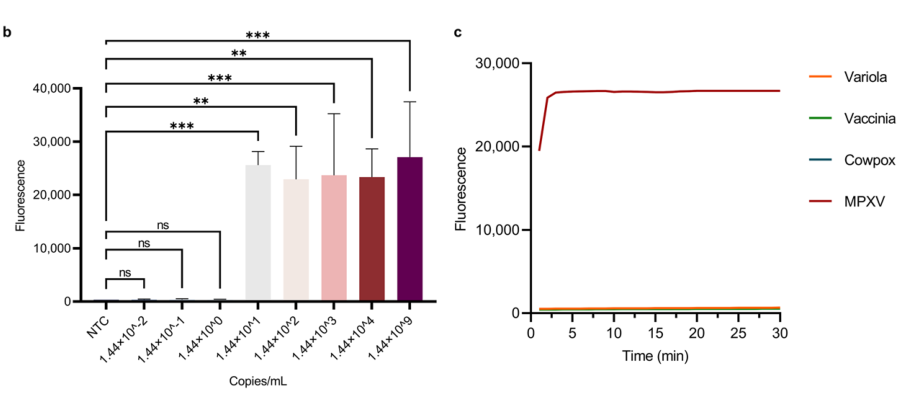
Figure 2 demonstrates the performance evaluation of the MIRA-CRISPR-Cas13a-MPXV fluorescence detection method. Figure b shows the results of the sensitivity test. (** indicates P < 0.01; *** indicates P < 0.001; ns indicates no statistically significant difference, P < 0.05 considered statistically significant). Figure c displays the reaction curves comparing MPXV with three other orthopoxvirus plasmids using the CRISPR-Cas13a system.
In clinical validation, the team tested 202 clinical MPXV samples and 104 interference samples. Results showed a high consistency with qPCR, with a Kappa value of 0.994, and importantly, the system successfully identified three true positives missed by qPCR, proving its comparable diagnostic performance and superior sensitivity. In addition, a fluorescent PCR-based typing system was developed, capable of distinguishing Clade I and II within 40 minutes, with no cross-reactivity with other orthopoxviruses — such as variola, cowpox, or vaccinia viruses — further enhancing virus tracking and surveillance.
A notable highlight of this method is its compatibility with multiple sample types, including rash fluid swabs, throat swabs, anal swabs, serum, and plasma. When combined with lateral flow strips, results are visually interpretable at room temperature within 5 minutes post-reaction, eliminating the need for specialized reading equipment. This truly achieves "sample-to-result" convenience, providing an effective solution for frontline healthcare and emergency response settings.
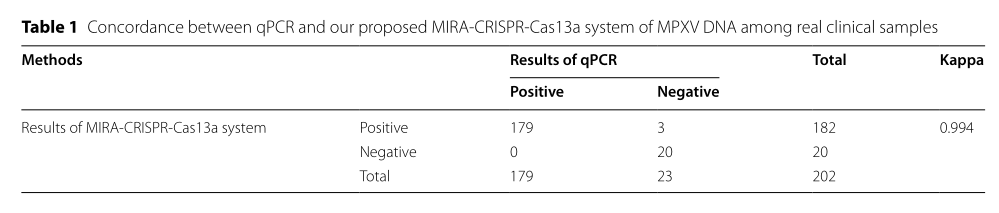
Table 1 presents the consistency analysis between qPCR and the MIRA-CRISPR-Cas13a system in real clinical sample testing.
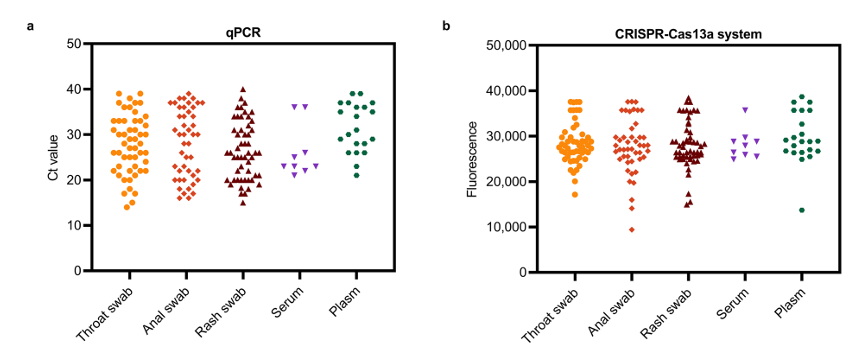
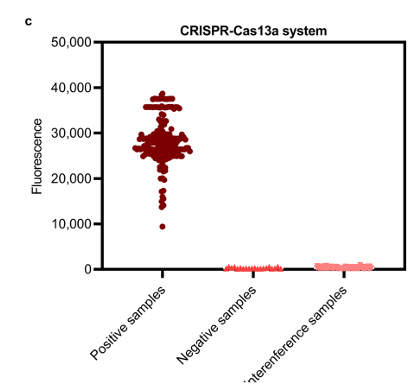
Figure 3 compares the results of clinical sample testing using qPCR and the MIRA-CRISPR-Cas13a method: a: Ct values from qPCR; b: Fluorescence intensity of clinical samples (MIRA-CRISPR-Cas13a detection); c: Fluorescence intensity of positive, negative, and interfering samples (MIRA-CRISPR-Cas13a method)
This research was supported by grants from the Social Development Project of Jiangsu Provincial Science and Technology Department, Nanjing Science and Technology Plan Project, the Graduate Research and Innovation Projects of Jiangsu Province, and the Talent Lift Program of The Second Hospital of Nanjing. Suzhou TianLong R&D experts were deeply involved throughout the project — from reagent preparation and system optimization to sample validation — contributing significantly to key processes such as complex sample handling, system integration, and clinical verification. This reflects Suzhou TianLong’s integrated capabilities in nucleic acid technology platforms, CRISPR-based engineering, and POCT product development.
As a leading molecular diagnostics company in China, Suzhou TianLong remains committed to integrating cutting-edge technologies with clinical needs. We continue to drive the transformation of CRISPR applications in infectious disease detection, respiratory pathogen monitoring, cancer screening, and maternal & child health. This success in developing a monkeypox detection system not only enhances China’s technological independence in epidemic response but also offers a portable, efficient, and low-cost solution to strengthen global public health defenses.
Looking ahead, Suzhou TianLong will continue accelerating the clinical implementation and international collaboration of its CRISPR solutions, driving more lab-developed technologies into practical field use — safeguarding global health with science and speed.

